- Find A Medical Provider
- Auto Injuries
- Common Injuries
- Medical/Pharmaceutical
- Types of Medical Injuries
- Malpractice Injuries
- Drug and Medical Device Injuries
- Drugs and Devices Linked to Cancer
- Opioid Addiction
- Drugs and Devices Known to Cause Injury
- 3M Combat Arms Earplugs – Hearing Loss
- Accutane
- Aciphex
- Actonel
- Actos
- Adderall and Ritalin
- Advair
- Aldara (Imiquimod)
- Alli
- Ambien
- Amiodarone
- Anzemet
- Aptivus
- Aranesp
- Arava
- Atorvastatin
- Avandia
- Benicar
- Birth Control Medication
- Blood Thinners
- Essure
- Fosamax (Alendronate Sodium)
- Gadolinium-Based MRI Contrast Agents
- Granuflo
- Hernia or Surgical Mesh Injuries
- Hydroxycut
- Inferior Vena Cava Filters
- Invokana Toe and Foot Amputations
- Ketek
- Levaquin
- Lipitor
- Mirapex
- Neurontin
- Onglyza
- Over-the-Counter Medications
- OxyContin
- Paxil
- Power Morcellators
- Pradaxa
- Propecia
- Reglan
- Talc Powder
- Trasylol
- Valsartan
- Viagra
- Xolair
- Zelnorm
- Zoloft
- Work Injuries
- Sports Injuries
- Marketing Services
- Blog
List your practice on InjuredCare | Log in / Sign up
Inferior Vena Cava Filters
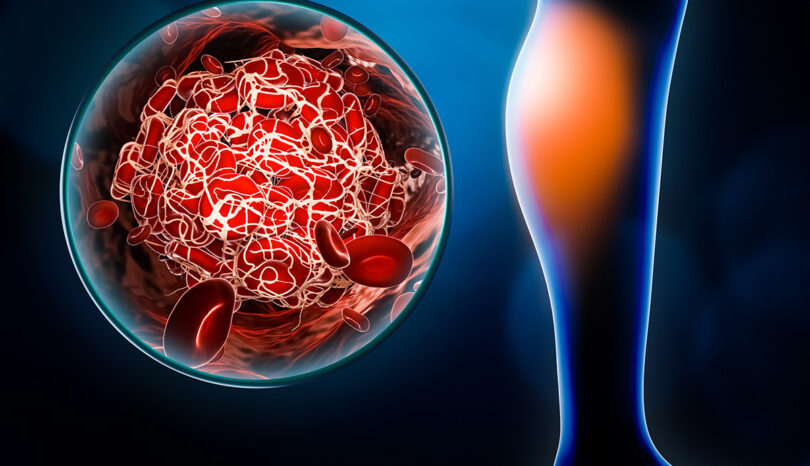
Inferior vena cava (IVC) filters are small, cone-shaped devices that may be surgically implanted near your kidneys to impede blood clots that have broken loose from one of the deep veins in your leg (a condition known as deep vein thrombosis). IVCs are designed to prevent those clots from reaching your lungs or heart. A medical professional will customarily implant the IVC filter using a catheter through a small incision in your neck or groin.
IVCs are generally not prescribed for individuals with low risk of blood clots. They typically are reserved for situations where anticoagulation is difficult.
According to the U.S. Food and Drug Administration (FDA), a significant number of patients who have received an IVC filter have reported adverse side effects, including:
- Movement or repositioning of the filter after insertion
- The detachment of parts of the filter after insertion
- Tears developing in the filter
- The failure or collapse of the filter
Most medical professionals believe that any problems with the IVC arise because the filters are left in the body longer than necessary. Thus far, though, manufacturers have not developed ways to easily retrieve the filters, as they tend to become overgrown with new cells in a matter of weeks.
First developed about 50 years ago, IVCs have come under increased scrutiny in the last decade, as the FDA has issued communications identifying potential health risks. The product is still approved for use and sale.