- Find A Medical Provider
- Auto Injuries
- Common Injuries
- Medical/Pharmaceutical
- Types of Medical Injuries
- Malpractice Injuries
- Drug and Medical Device Injuries
- Drugs and Devices Linked to Cancer
- Opioid Addiction
- Drugs and Devices Known to Cause Injury
- 3M Combat Arms Earplugs – Hearing Loss
- Accutane
- Aciphex
- Actonel
- Actos
- Adderall and Ritalin
- Advair
- Aldara (Imiquimod)
- Alli
- Ambien
- Amiodarone
- Anzemet
- Aptivus
- Aranesp
- Arava
- Atorvastatin
- Avandia
- Benicar
- Birth Control Medication
- Blood Thinners
- Essure
- Fosamax (Alendronate Sodium)
- Gadolinium-Based MRI Contrast Agents
- Granuflo
- Hernia or Surgical Mesh Injuries
- Hydroxycut
- Inferior Vena Cava Filters
- Invokana Toe and Foot Amputations
- Ketek
- Levaquin
- Lipitor
- Mirapex
- Neurontin
- Onglyza
- Over-the-Counter Medications
- OxyContin
- Paxil
- Power Morcellators
- Pradaxa
- Propecia
- Reglan
- Talc Powder
- Trasylol
- Valsartan
- Viagra
- Xolair
- Zelnorm
- Zoloft
- Work Injuries
- Sports Injuries
- Marketing Services
- Blog
List your practice on InjuredCare | Log in / Sign up
Arterial Blockage – ST-Elevation Myocardial Infarction (STEMI)
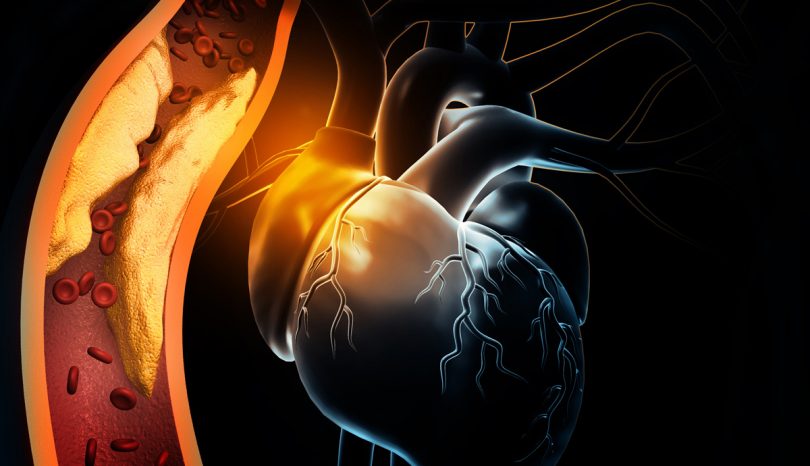
An STEMI (ST-elevation myocardial infarction) is the medical term for a serious heart attack. It's typically associated with a blockage in one of the main arteries leading into the heart. In most cases, it's an indication that the patient suffers from artherosclerosis, a form of coronary artery disease.
The diagnosis of a STEMI comes from an electrocardiogram. Individuals suffering from acute STEMI have a significant risk of sudden or massive heart attack caused by arrhythmias, such as ventricular fibrillation. Successful treatment includes defibrillation and CPR (cardiopulmonary resuscitation) to reestablish a normal heart rhythm.
Symptoms of a STEMI
Indications that you may be experiencing an ST-elevation myocardial infarction include:
- Shortness of breath or difficulty breathing
- Chest pains
- Nausea or vomiting
- Sweatiness unrelated to current temperatures
- Dizziness or lightheadedness
- Heart palpitations (a greater sense of the awareness of the beating of your heart)
- Anxiety
The early signs of STEMI may be similar to those of heartburn or even indigestion. It's best to seek treatment for any abnormality.
Treatment of STEMI
Because of the serious nature of a STEMI, it's typical to take more radical steps in response. Though clot-busting drugs may be administered, an angioplasty or stent is often one of the first measures. With an angioplasty, a "balloon" is surgically implanted and inflated, opening the blocked artery. The optimal time period for the angioplasty is within 90 minutes of the first signs of the STEMI.
If the artherosclerosis is too severe, an angioplasty may not work. In such cases, a patient may require open heart surgery, typically in the form of a coronary artery bypass graft, also known as "bypass surgery."